The beginning of my time at UCLA was pretty rough. I spent my first year in and out of the hospital battling Ulcerative Colitis, an autoimmune disease that first reared its ugly head shortly after I turned 16. My second and third years were likewise spent away from campus due to the COVID-19 pandemic. I was admitted as a Materials Science and Engineering student but quickly found my classes on the chemical properties of materials much more interesting than the ones on their physical properties. In particular, I took organic chemistry with Hung V. Pham, during which I realized that Ochem- and the world of molecules- simply clicked better in my brain than analyzing stress-strain curves in my engineering classes. If any UCLA undergrads are reading this, take (at least) one of Hung’s classes, he’s one of the best educators I’ve ever had the pleasure of learning from. After all, I had chosen MatSci because I wanted to make new stuff, and I saw understanding the chemical properties of materials as much more important for that. In a move that confused my engineering advisor, I switched my primary major from Materials Engineering to Materials Chemistry (I also double majored in Political Science), with a concentration on organic materials. Although my choice to leave the school of engineering vexed quite a few people, I don’t regret it one bit. I love the world of atoms and molecules and the degree path I took was perfect for my interests at the time. However, I still had a major problem: I was gunning for the prestigious Departmental Scholars Program, in which students can stay an extra year as an undergrad student while taking grad-level classes, do research in the field of their choice, and receive a Master’s degree for only $13k. I was full steam ahead with my grades, receiving nearly straight As (including a personal-best 97% in Ochem 2) and recovering from the dismal 2.7 GPA I had earned when I was sick, but one problem stood in my way: I had to be doing research with a tenured faculty member.
With no previous research experience in STEM, I was entering my senior year desperate to find a lab when I sent an email to my inorganic and materials chemistry professor, Richard Kaner (Ric) explaining my situation. He said that due to COVID-19 measures, it was difficult to take on new undergrads, but nonetheless found me a spot helping out a 5th-year PH.D. student, Mackenzie Anderson (Mac). I was quite fortunate and happy with the situation, as Ric is one of the preeminent experts in the world on quite a few topics, including composite materials of conducting polymers, chemical sensors, memory devices, high-density electronics, actuators, transparent conductors, superhard materials, thermoelectric materials, and graphene. Plus I had- and still have- tremendous interest in new materials that can make the world a more sustainable place, so working with Mac on her nanofiltration (desalination) membranes was a good place to start.
At this point, I want to say I don’t think I did the whole research thing right. There wasn’t any problem with the quality of the work I produced, but I was too distracted by other things- classes, my internship, sports (I played on UCLA’s Lacrosse Team), making up for the lost 2 years of socializing due to COVID, other clubs at UCLA, my girlfriend- to ever truly immerse myself in the research work I was doing. I have a personal rule of doing everything 110% and I broke that rule. I logged an average of roughly 10 hours per week in the lab (and most of that was skewed by longer hours during the summer and spring), but to be honest, I probably should’ve spent at least twice that there. Maybe then I would’ve been immersed enough to enjoy the work more. Anyways, back to the story.
When I started in June 2021, Mac was working on getting the methodology right for getting the membranes to stick and then lift properly. The work involved synthesizing various polymeric compounds, applying them to a rather large glass slide, and then soaking them in various reagent baths. This was my favorite part of the stuff I did with Mac, even though some of the reagents gave me extraordinary headaches (and burned holes in several garments). Here’s what they looked like:
Another part of the membrane work was testing them in pressure chambers with various-sized dyes. I don’t have any pictures of the chambers but the dyes looked like this and stained everything:
But I wanted to run before I could walk. So I did what any headstrong 20-year-old with access to a lab full of chemicals would do and started doing experiments on my own. During a biomaterials class (by far the most difficult I took at UCLA) I did a project with another Chem/Matsci student named Jongha on a newly emerging class of nanoparticles called quantum dots, which his father, a researcher at Samsung, was working on (now used for QLED displays). In our project, we examined a novel approach of using QDs for inexpensive bioimaging. I liked the idea; the main use of them at the time was for cell labeling at the microscopic level, but I thought they could be more useful as a low-cost contrast dye. The cool thing about QDs is that they have tunable electron band gaps, which means you can essentially control what wavelength of light they fluoresce. My idea was that if you polymerized QDs with magnetic nanoparticles into small nanostructures, you could use a magnetic field to alter their size (like an expandable ball toy) and see what was going on in different tissues. Here are some constraints that I figured out this experiment would require:
The QDs needed to be cheap, easy to make, and biocompatible.
The magnetic nanoparticles had to be polymerizable, cheap, easy to make, and biocompatible.
The nanostructures had to fit through certain cellular membranes, which means they needed to be size-tunable.
Ok, step 1. The project I did with Jongha utilized CdSe quantum dots, which aren’t the cheapest, so I went back to my foundations and realized that organic molecules with significant pi-bond character had pretty decent fluorescence. Thus I decided using carbon quantum dots (CQDs) was the way to go. During my time working with Mac, I became familiar with an incredibly cheap polymer that you could buy in varying lengths, polyethylene glycol (PEG), and thought it would be an excellent candidate for the ligands. I then fiddled around in the lab to try and make a monomer that looked something like this, and then have it undergo elimination reactions to produce pi bonds:
Here is the synthetic route I took:
10 mmol Allyl bromide is added dropwise to a solution of 1 mmol d-glucose + 12.5 mmol KOH in toluene.
Reflux for 12h. Filter and evaporate filtrate.
Dissolve in CHCl3 and wash with water. Dry/concentrate under vacuum.
Purify with column chromatography; elutent: CHCl3, then CHCl3:MeOH (100:1)
5 mmol mCPBA added to purified product in toluene.
Stir for 24h at rt.
Filter precipitate out, then wash organic layer with NaHCO3 (25mL twice) then Na2CO3 (25mL twice).
Purify with column chromatography; elutent: CHCl3, then CHCl3:MeOH (100:4)
Add 5 mmol Boc2O to 5 mmol amino-PEG in H2O
Add 1 mmol filtered product to 5 mmol a-PEG in H2O / 10 mmol NaOH.
Stir for 8h at rt.
Neutralize with citric or trifluoroacetic acid, separate organic layer, and wash with DI water.
The big problem with this is that I found that I couldn’t get the ethers to undergo elimination, which made the pi bonds essentially nonexistent. Back to the drawing board.
After some extensive digging on CQDs, I found an alternative bottom-up method that was even cheaper than my previous attempts. It required little more than some PEG, some sugar, and a microwave oven. This new methodology required getting the sugar to dissolve in the PEG, which presented a challenge since the solubility of glucose in PEG was so low, so I added a third, cheap ingredient: Water. Unfortunately, the only 500W oven I had access to was marked for food only, so I used an 850W one, which made the ratios provided in Zhu et al’s paper all but useless. But ever the experimentalist, I tried to find a way to make it work. The main problem I was having was due to the increased power of the microwave, which boiled the water too quickly for pyrolysis to occur, instead crashing out the sugar and burning it (and my hand, on a few occasions). I got a warning from a professor that was passing by, thinking I was baking in the lab, since it smelled so sweet. Eventually, I was able to get a ratio of water, glucose, and PEG that worked and was thin enough to use. Here’s what I affectionately referred to as my glo-PEG:
The luminescence was faint, but it was there! PEG normally glows blue, so I knew I had something. I then tried a couple of different solvents to separate the CQDs out of the glo-PEG and got it isolated in water. I wish I could remember the exact combinations of everything off the top of my head, but unfortunately, it’s all in a lab notebook in the Molecular Sciences building.
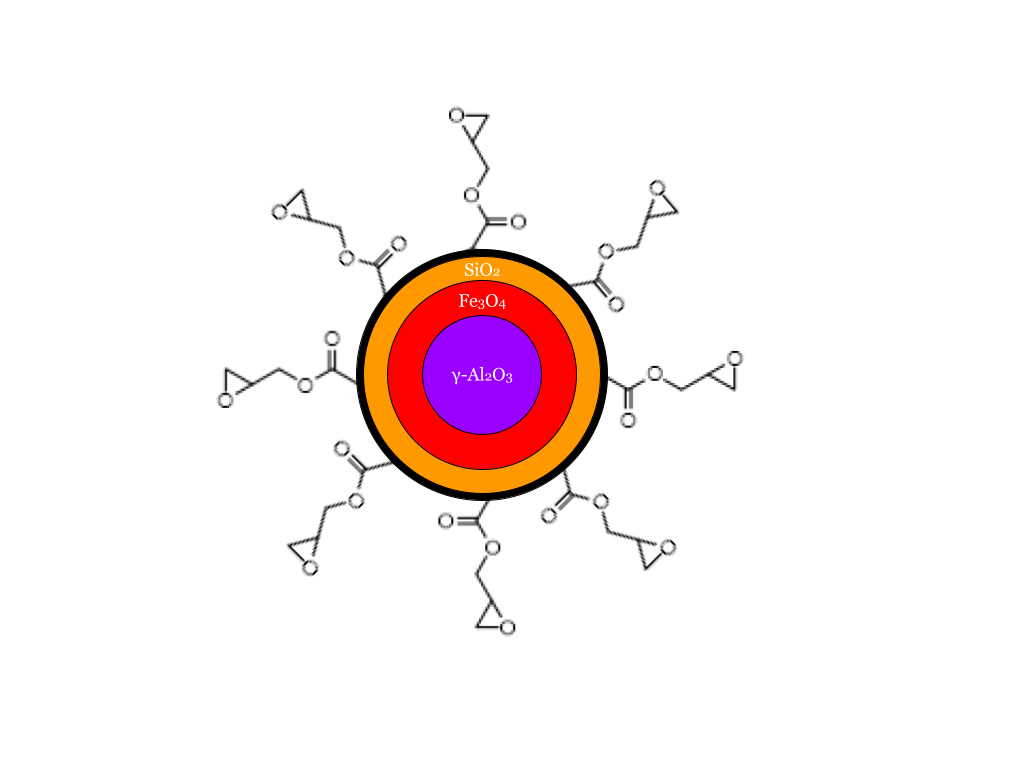
Step 2. At this point, I had shifted my efforts almost entirely to my own research and submitted my proposal for entry to the Departmental Scholars Program. With my CQDs ready, I proceeded to work on the magnetic nanoparticles. These were quite a bit more complex to make, but here’s a figure I made to explain it:
The problem with magnetic polymers such as PANiCNQ and TCNE-Mn is that they don’t like to behave under standard conditions: They are low-strength and either need to be put under a magnetic field for a long time to self-order or cooled to around -200C to magnetize. Alternatively, magnetite exhibits pretty high magnetic strength and has fairly well-known rheological properties. The challenge was then how to get magnetite into a polymerizable form and control how strong their magnetism was. I went back to Google Scholar, and soon came across an article that demonstrated ɣ-Al2O3 had a similar crystal structure as magnetite and thus would be a suitable substrate to grow magnetite on. Although ɣ-Al2O3 isn’t as cheap as normal alumina is to buy off-the-shelf, it’s not expensive nor difficult to make, so I was satisfied with that layer. This is the synthetic route I used:
2g urea is added to 35g Al(NO3)3⋅9H2O dissolved in 35g DI water. (1:13 Al3+:urea)
Stir for 1h at rt.
Filter impurities out
Heat at 90°C for 12h in an oil bath until pH reaches ~6.
Heat another 3h until a transparent gel.
Dry in oven at 300°C for 3h to form ɣ-Al2O3 nanoparticles. (100% yield is ~4.75g)
The procedure of “growing” magnetite on the alumina (in quotes because the process is closer to doping than crystal growth) was pretty straightforward:
0.5g ɣ-Al2O3 nanoparticles are added to 10g water containing 0.0125g CTAB surfactant.
(1:1.87 mol ratio) FeSO4⋅7H2O:FeCl3 and 0.26g 25% NH4OH is mixed with alumina/solvent.
Stir for 2h under a nitrogen atmosphere at 70°C.
Treat emulsion with 1.3g of 2M HNO3 for 15m.
Wash magnetically until the solution is neutral.
Add 0.4M citric acid and stir at 100 rpm overnight.
Wash magnetically until the solution is neutral.
From here, the path to polymerization was pretty simple (or so I thought). By 2021, there had been several methods devised to coat magnetite in silica for biomedical applications, so I chose one I had some familiarity with:
Mix 24g DI water, 4g EtOH, 0.196g CTAB at 60°C.
Add 0.5g ɣ-Al2O3/Fe3O4 nanoparticles to the solvent under continuous stirring.
Add 0.26g 25% NH3 solution until pH is 9-11 under continuous stirring.
Add 0.5g TEOS dropwise to the mixture under continuous stirring.
Heat at 60°C for 2h until the black solution fades.
Wash magnetically with EtOH and DI water 5 times.
After a significant amount of trial and error getting all the crystallization conditions correct, I had some pretty strong magnetic nanoparticles! You can’t see it super well in this photo, but they were in an aqueous form here:
Here comes the part where it all started to go wrong: Glycidyl methacrylate (GMA), the outer layer on the magnetic nanoparticles, has an epoxy group on its end. I attempted the following procedure to attach the GMA to the silica layer:
Preheat ɣ-Al2O3/Fe3O4/SiO2 nanoparticles in water bath at 75°C.
Add 60g water, 0.2g ɣ-Al2O3/Fe3O4/SiO2 nanoparticles, 0.4g GMA into flask.
Add 0.012g V-50 cationic azo initiator to begin seeded polymerization on SiO2.
Stir for 24h at 100 rpm under nitrogen.
But no matter what amount of the V-50 I used, the GMA self-polymerized instead of binding to the silica. I had devised a simple polymerization method for combining the CQDs and magnetic nanoparticles that I hoped to use:
Add 5 mol NaOH to 1 mol monomer A.
Add deprotonated/initiated monomer A to monomer B in DI water.
Stir at 100 rpm for 24h under nitrogen at 75°C or stir initially then allow it to react in a magnetic field for 24h under nitrogen at 75°C.
Sol-gel methods may be tried here.
Neutralize with citric acid.
Magnetically separate.
However, the GMA wouldn’t behave. In pretty much all experiments, it stuck to itself instead of the magnetic nanoparticles or the CQDs. Several different technique attempts later, I still had two separate monomers that were all but useless without the other.
At this point, it was nearly graduation time. Combined with other personal factors that arose in March 2022, the frustration set in and soon enough, I was looking at other grad school programs, despite having been admitted to the Departmental Scholars Program for Materials Chemistry. I’ve always considered myself a creative, big-picture thinker, and I by then had developed the belief that I would not be able to have a large enough effect on humanity just by doing scientific laboratory research. I wanted to figure out how to scale labor, not necessarily just use my own.
During my internship at Terrascale, I became interested in the field of complexity science, in which computational models are used to describe how systems actually behave (as opposed to models that predict how they should behave). The biggest problem was that the only program completely dedicated to Complexity Science was at Arizona State University, and I didn’t think ASU was the place for me. I also applied to a few other programs, but I found out Columbia University had a seminar on Complexity Science led by the new Academic Director of the Information and Knowledge Strategy program, Christoph Meinrenken (who would later be my research advisor). IKNS was pretty close to what I wanted to learn and seemed cool and innovative, Columbia was a good name to fall back on, and NYC seemed like a good place for personal growth, so I applied.
And that’s where this chapter ends and the next begins. I withdrew from DSP the day before graduation and was admitted to Columbia a few days later. More on that in a future post.